Research led by the HydroMet group is driving innovation in lithium-ion cathode chemistry, potentially opening a pathway to a new generation of cobalt-free, energy-dense batteries for maritime application
The HydroMet research group at McGill University, led by principal investigator Prof. George P Demopoulos, is part of McGill’s sustainability initiative. Over the past several decades, the group has been conducting research into sustainable renewable energy generation and storage systems, specifically Li-ion batteries (LIBs) and solar cells.
Currently it is estimated that 40% of a Li-ion battery cell’s cost is associated with the cathode. Therefore, over the past few years, experts in the HydroMet research group have devoted their attention to the development of a cathode made of lithium, iron and silicate (LFS), which is not only cost-effective but also an inherently safe and high-energy-density cathode material. This silicate research project was funded by Canada’s Natural Sciences and Engineering Research Council (NSERC) and supported by Hydro-Québec, a globally renowned key player in battery development.
Chemistry of choice
Among all the technologically mature battery systems, lithium-ion comes closest to internal combustion engines in terms of energy and power density – especially when compared with other conventional rechargeable battery solutions. Since the commercialization of lithium-ion batteries in the early 1990s, lithium-ion has become the dominant chemistry for portable devices. It has also penetrated the transportation market, becoming the chemistry of choice in most hybrid and electric road vehicles.
The development of BEVs in the automotive segment has advanced rapidly. But due to market demand for greater ranges and more energy efficiency, a shift to higher-energy-density LIBs has become necessary.
The energy density of a lithium-ion battery is dependent on the anode (negative electrode), cathode (positive electrode) and electrolyte materials. The cathode is currently a notable bottleneck in the development of LIBs; today’s solutions have a low energy density, high cost, sluggish charging and discharge rate, and low voltage. Therefore the HydroMet research group believes that effort should be devoted to the development of high-power and energy-density cathodes.
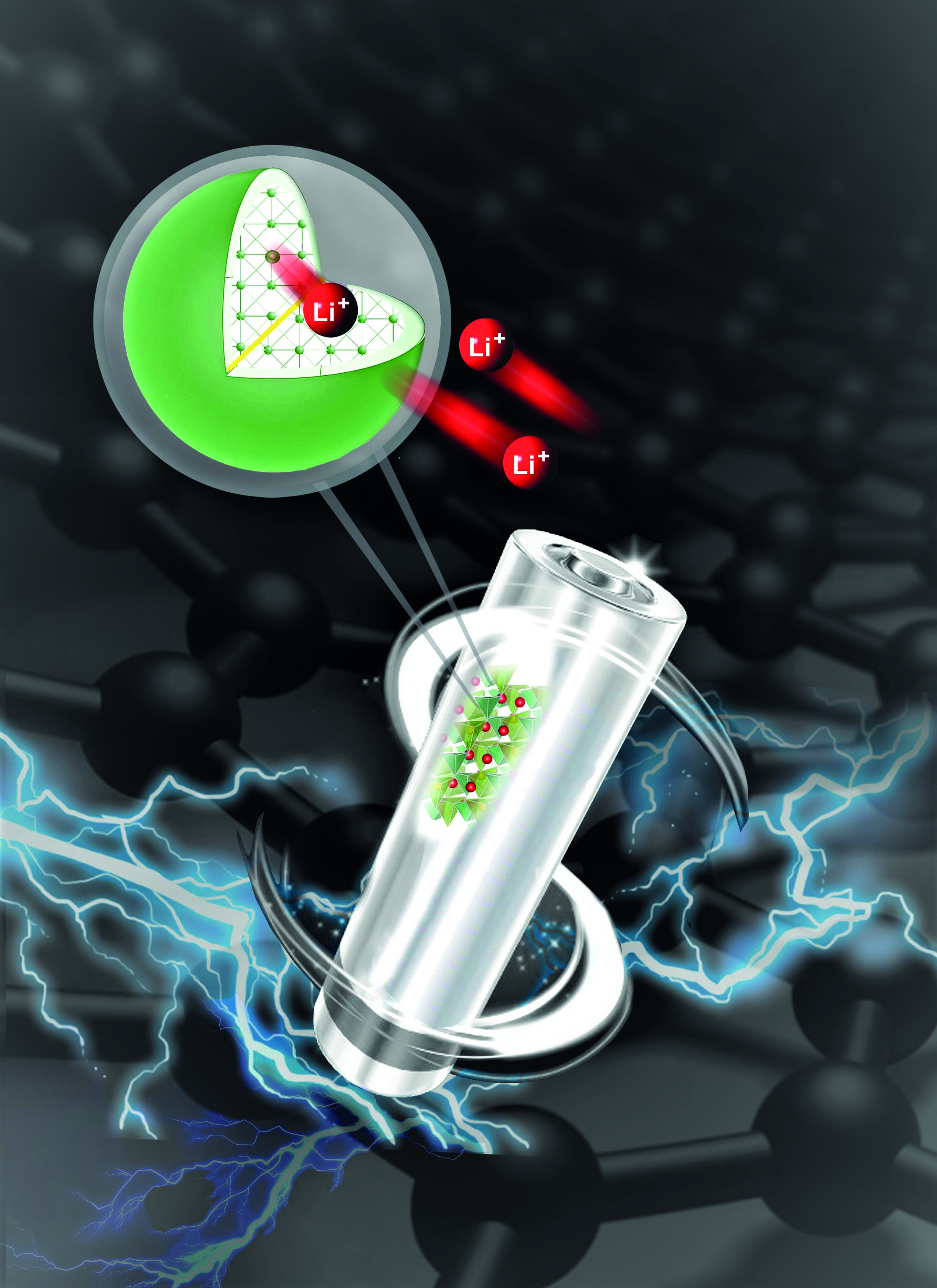
Source material
Cobalt is an expensive metal used widely in the cathode in commercial applications. About 60% of the world’s cobalt supply comes from the Democratic Republic of Congo. The mining of cobalt has been linked to human rights abuses, corruption, environmental destruction and child labor.
Many companies would, therefore, like to considerably reduce or eliminate cobalt in lithium batteries, but experts are yet to find an alternative that offers the same efficiency. Well-known BEV manufacturer Tesla recently announced that it is currently working on the development of cobalt-free batteries, which will be based on lithium iron phosphate (LFP).
Intriguingly, LFS, the material of interest at the HydroMet laboratory, has double the theoretical capacity of LFP and can hold its capacity over several charge-discharge cycles. In practice, however, this hasn’t been achieved, due to poor intrinsic conductivity, resulting in slow movement of the electron and ions while charging and discharging the battery.
Interestingly, the group’s recent study focusing on the specific phase (crystal structure) of this cathode shows that these affordable materials could prove key for improving battery performance. Researchers adopted a polymer coating procedure that could enable iron and silicon – two of the world’s most abundant elements – to be used instead of cobalt. The results were analyzed and validated at the Canadian Light Source (CLS) at the University of Saskatchewan.
As it happens, commercially available LFP suffered from the same issues during its research phase. That was resolved using carbon coating for each of the individual nanocrystals. Conventionally, individual cathode particles are coated with a high-temperature carbon coating. But in the case of the low-temperature phase of LFS, this would completely change its structure, which is usually highly unstable and delivers poor long-term cycling performance. The team was therefore keen to utilize an alternative technique to coat the individual nanoparticles to improve conductivity while keeping their original structure.
Researchers used a polymer known as PEDOT, which is electronically conductive and can essentially coat the nanoparticles similarly to widely used carbon coating methods. This process was easier said than done. Figuring out a way to apply the coating to the surface of the nanocrystals took almost two years due to difficulty in keeping the original structure of the nanocrystals. Once the coating was successfully applied, LFS nanocrystals showed a surprisingly big jump in performance over carbon coating.
Research validation
To validate the research, cathodes were sent to CLS, where they were tested using CMCF beamlines and spectromicroscopy. These high-resolution testing techniques enabled researchers to dig deeper and begin to explain why the PEDOT coating treatment and the subsurface iron-rich layer improved the level of performance so much. Although there is still work to be done to understand why and build on this finding, this high-density cathode material opens up another pathway for cobalt-free batteries.
The PEDOT coating method has the potential to unleash the full theoretical capacity of LFP, enabling it to become a commercially viable cathode for BEVs and a power source for various use cases including marine applications.
A battery essentially made of iron (rust) and sand (silica) could even become a possibility. With this development, the industry could look forward to cheaper batteries and mass electrification of transportation systems across the globe.
 By Majid Rasool, postdoctoral fellow, McGill University
By Majid Rasool, postdoctoral fellow, McGill University
Images: McGill University, Montreal, Quebec, Canada


